Overview
Cardiol Therapeutics (NASDAQ:CRDL,TSX:CRDL) is a clinical-stage life sciences firm targeted on the analysis and scientific improvement of anti-inflammatory and anti-fibrotic therapies for the therapy of coronary heart illness. The corporate’s lead small molecule drug candidate, CardiolRx™ (cannabidiol) oral resolution, is pharmaceutically manufactured and in scientific improvement to be used within the therapy of coronary heart illness. It’s acknowledged that cannabidiol inhibits activation of the inflammasome pathway, an intracellular course of recognized to play an vital function within the improvement and development of irritation and fibrosis related to myocarditis, pericarditis and coronary heart failure.
Cardiol has obtained Investigational New Drug Utility authorization from america Meals and Drug Administration to conduct scientific research to judge the efficacy and security of CardiolRx in two ailments affecting the center: (i) a Part II multi-center open-label pilot examine in recurrent pericarditis (the MAvERIC-Pilot examine; NCT05494788), an inflammatory illness of the pericardium which is related to signs together with debilitating chest ache, shortness of breath, and fatigue, and ends in bodily limitations, lowered high quality of life, emergency division visits, and hospitalizations; and (ii) a Part II multi-national, randomized, double-blind, placebo-controlled trial (the ARCHER trial; NCT05180240) in acute myocarditis, an vital reason for acute and fulminant coronary heart failure in younger adults and a number one reason for sudden cardiac demise in individuals lower than 35 years of age.
Cardiol can also be growing a novel subcutaneously administered drug formulation of cannabidiol supposed to be used in coronary heart failure – a number one reason for demise and hospitalization within the developed world, with related healthcare prices in america exceeding $30 billion yearly.
Firm Highlights
- Lead Asset in Medical Growth: CardiolRx™, oral drug candidate, in Part II trials for recurrent pericarditis and acute myocarditis.
- Scientific Rationale: Compelling proof demonstrating the anti-inflammatory and antifibrotic properties of CardiolRx in myopericardial ailments.
- Progressive Analysis: Advancing the event of CRD-38, a novel proprietary subcutaneously administered pharmaceutical supposed to be used in coronary heart failure.
- CRD-38 Optimistic Examine Outcomes: Optimistic examine outcomes demonstrating CRD-38 subcutaneous formulation slowed will increase in physique weight and coronary heart weight, and prevented will increase in key cardiac inflammatory and transforming markers in a mannequin of coronary heart failure with preserved ejection fraction.
- Broad Exclusivity Safety: Complete mental property portfolio. Eligible to pursue FDA orphan drug and EMA orphan drugs designations for CardiolRx.
- Management: Skilled administration group, board of administrators, and scientific advisory board, with in depth experience in growing therapeutics for inflammatory coronary heart illness.
- Robust Monetary Place: Debt-free and well-capitalized to realize company milestones into 2026.
Key Initiatives
Orphan Drug Program for Recurrent Pericarditis
In November 2023, Cardiol introduced that it has exceeded 50 % of the affected person enrollment goal for the company-sponsored Part II open-label MAvERIC-Pilot examine investigating the tolerance, security and efficacy of CardiolRx in sufferers with recurrent pericarditis. The examine may also assess the development in goal measures of illness, and through an extension interval, assess the feasibility of weaning concomitant background remedy together with corticosteroids, whereas taking CardiolRx.
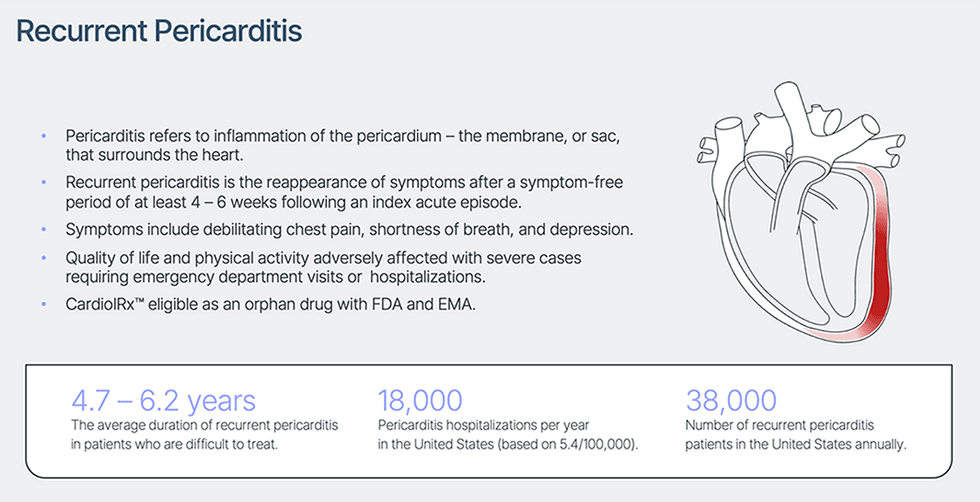
Pericarditis refers to irritation of the membrane or sac that surrounds the center (the pericardium) that’s most often triggered by a viral an infection. Recurrent pericarditis is the most typical complication following an preliminary acute episode of pericarditis, and sufferers might have a number of recurrences. Signs embrace debilitating chest ache, shortness of breath, and fatigue, leading to bodily limitations, lowered high quality of life, emergency division visits, and hospitalizations. Rare however life-threatening issues related to pericarditis embrace a big accumulation of pericardial fluid, scarring, and constriction of the center which can restrict coronary heart perform. The illness is recognized in 0.2 % of all cardiovascular in-hospital admissions and is liable for 5 % of emergency room admissions for chest ache in North America and Western Europe. Recurrent pericarditis is the re-appearance of signs after a symptom-free interval of a minimum of 4 – 6 weeks following an preliminary acute episode of pericarditis. These recurrences seem in 15 % to 30 % of acute circumstances and normally inside 18 months. Moreover, as much as 50 % of sufferers with a recurrent episode of pericarditis expertise extra recurrences. Customary first-line medical remedy consists of non-steroidal anti-inflammatory medicine or aspirin with or with out colchicine. Corticosteroids corresponding to prednisone are second-line remedy in sufferers with continued recurrence and insufficient response to standard remedy.
The one FDA-approved remedy for recurrent pericarditis, launched in 2021, is usually used as a third-line intervention in sufferers with a 3rd or fourth recurrence. The variety of circumstances of sufferers looking for and receiving therapy for recurrent pericarditis yearly within the US is estimated at 38,000. Hospitalization as a consequence of recurrent pericarditis is usually related to a 5 – 8 day size of keep and value per keep is estimated to vary between $20,000 and $30,000 in america.
The Part II MAvERIC-Pilot examine is predicted to enroll 25 sufferers at scientific facilities in america focusing on pericarditis care. The protocol has been designed in collaboration with thought leaders in pericardial illness. The examine chairman is Dr. Allan L. Klein, director of the Heart of Pericardial Illnesses and professor of drugs, on the Coronary heart and Vascular Institute on the Cleveland Clinic. The first efficacy endpoint is the change, from baseline to eight weeks, in patient-reported pericarditis ache utilizing an 11-point numeric score scale (NRS). The NRS is a validated scientific instrument employed throughout a number of circumstances with acute and power ache, together with earlier research of recurrent pericarditis. Further endpoints throughout the extension interval embrace the NRS rating after 26 weeks of therapy, and adjustments in inflammatory marker C-reactive protein (CRP), a generally used scientific marker of irritation.
“Recurrent pericarditis is a debilitating inflammatory coronary heart illness related to signs that adversely have an effect on high quality of life and bodily exercise. Outcomes of the MAvERIC-Pilot examine will help in additional understanding the therapeutic profile of our lead investigational drug on this situation and inform the design of a pivotal Part III scientific trial to underpin the potential regulatory approval of CardiolRx, which can also be being evaluated within the world ARCHER Part II trial in sufferers presenting with acute myocarditis,” stated David Elsley, Cardiol Therapeutics’ president and chief govt officer.
Within the US, an orphan drug designation is granted for prescribed drugs being developed to deal with medical circumstances affecting fewer than 200,000 individuals. These circumstances are known as orphan ailments. The task of orphan standing to a illness and to medicine developed to deal with it’s a matter of public coverage in lots of nations and has yielded medical breakthroughs that may not in any other case have been achieved. Within the US and the European Union, orphan medicine are eligible for accelerated advertising and marketing approvals and corporations growing orphan medicine sometimes obtain different incentives, together with a protracted interval of market exclusivity that may prolong over seven years, throughout which the drug developer has sole rights to market the drug.
Recurrent pericarditis is an orphan illness in america, thereby making CardiolRx eligible for orphan drug standing below the FDA’s Orphan Drug Designation program.
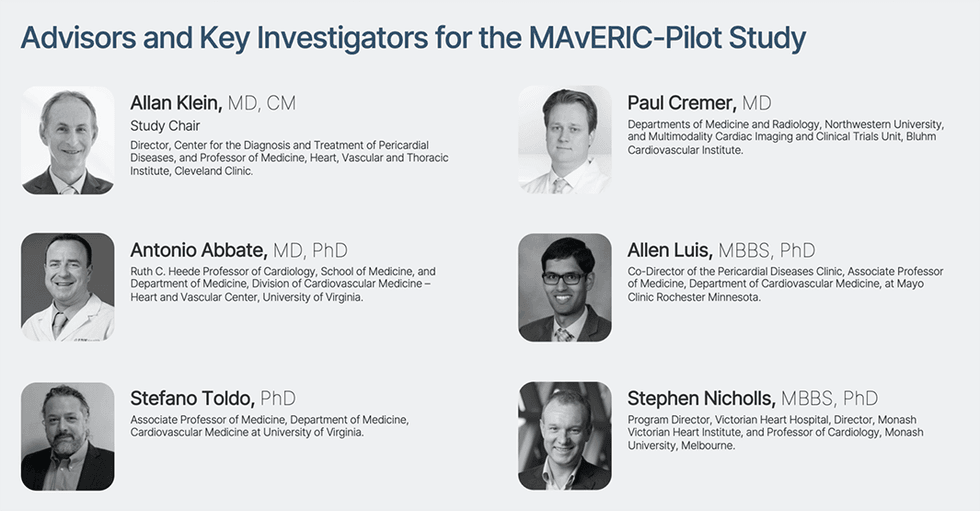
Unbiased advisors and key investigators comprising six extremely distinguished thought leaders in cardiology from the Cleveland Clinic, the Mayo Clinic, the Monash Victoria Coronary heart Institute, the College of Virginia, and Northwestern College have been established to design, oversee, and information Cardiol’s Part II MAvERIC-Pilot examine in sufferers with recurrent pericarditis.
Orphan Drug Program for Acute Myocarditis
In January 2024, Cardiol exceeded 50 percent patient enrollment in ARCHER, the corporate’s Part II multi-center, worldwide, double-blind, randomized, placebo-controlled trial designed to review the security and tolerability of CardiolRx, in addition to its impression on myocardial restoration, in sufferers presenting with acute myocarditis.
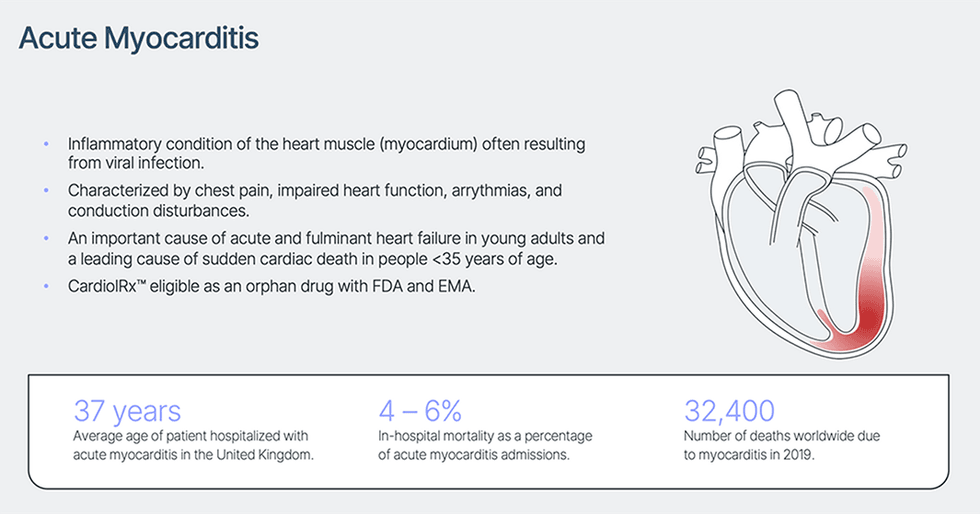
Myocarditis is an acute inflammatory situation of the center muscle (myocardium) characterised by chest ache, impaired cardiac perform, atrial and ventricular arrhythmias, and conduction disturbances. Though the signs are sometimes delicate, myocarditis stays an vital reason for acute and fulminant coronary heart failure and is a number one reason for sudden cardiac demise in individuals below 35 years of age. Though viral an infection is the most typical reason for myocarditis, the situation may outcome from bacterial an infection, generally used medicine and mRNA vaccines, in addition to therapies used to deal with a number of frequent cancers, together with chemo-therapeutic brokers and immune checkpoint inhibitors.
In a proportion of sufferers, the irritation within the coronary heart persists and causes decreased coronary heart perform with signs and indicators of coronary heart failure, and as such therapy relies on standard-of-care suggestions for coronary heart failure. This consists of diuretics, ACE inhibitors, angiotensin receptors blockers, beta-blockers, and aldosterone inhibitors. For these with a fulminant presentation, intensive care is usually required, with the usage of inotropic drugs (to extend the drive of the center muscle contraction). Extreme circumstances often require ventricular help units or extracorporeal oxygenation and will necessitate coronary heart transplantation.
There are not any FDA-approved therapies for acute myocarditis. Sufferers hospitalized with the situation expertise a median 7-day size of keep and a 4 – 6 % threat of in-hospital mortality, with common hospital cost per keep estimated at $110,000 in america.
“The US orphan drug program was efficiently utilized to speed up the primary FDA approval of CBD for the therapy of uncommon types of pediatric epilepsy, and important shareholder worth was created within the course of,” Elsley stated. “Given the mortality and important morbidity threat related to acute myocarditis, we imagine there’s a comparable alternative in pursuing an expedited improvement program of our CardiolRx pharmaceutical CBD formulation for this severe orphan illness which has no accepted commonplace of care.”
The first efficacy endpoints of the Part II ARCHER trial include extracellular quantity (ECV) and world longitudinal pressure (GLS). The secondary efficacy endpoint is left ventricular ejection fraction. Since individuals with acute myocarditis have impaired coronary heart perform, present therapy relies on standard-of-care suggestions for coronary heart failure. This consists of diuretics, ACE inhibitors, angiotensin receptors blockers, beta-blockers, and aldosterone inhibitors. For these with a extreme and sudden onset presentation, intensive care is usually required, with the usage of inotropic drugs (to extend the drive of the center muscle contraction) and sometimes, heart-lung bypass or ventricular help units. There may be in any other case no particular therapy for acute myocarditis though some sufferers have responded to immuno-suppressive remedy (azathioprine) together with steroids, however the knowledge usually are not conclusive sufficient for this to be the really helpful remedy.
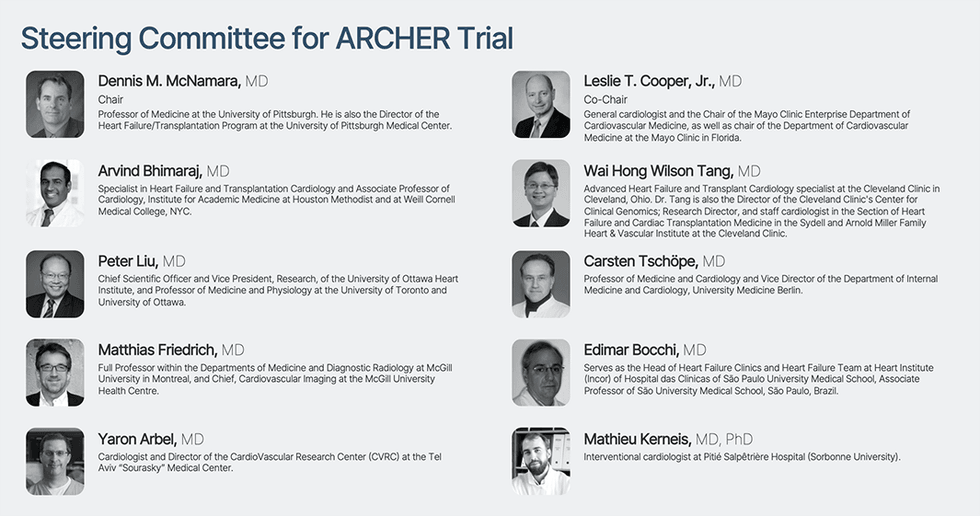
An unbiased scientific steering committee, comprising 10 extremely distinguished thought leaders in cardiology from the Cleveland Clinic, the Mayo Clinic, the Houston Methodist DeBakey Coronary heart and Vascular Heart, the College of Ottawa Coronary heart Institute, McGill College Well being Centre, the College of Pittsburgh Medical Heart, College Medication Berlin, Tel Aviv “Sourasky” Medical Heart, São Paulo College Medical Faculty, and Pitié Salpêtrière Hospital (Sorbonne College), has been established to design, oversee, and information Cardiol’s Part II multi-national ARCHER trial in acute myocarditis.
Cardiol Therapeutics’ Coronary heart Failure Program
Coronary heart failure impacts greater than 64 million individuals globally and related healthcare prices exceed $30 billion yearly within the US alone. Coronary heart failure is a power, progressive syndrome wherein the center muscle is unable to pump sufficient blood to fulfill the physique’s wants for blood and oxygen. Individuals with coronary heart failure endure from shortness of breath, fast coronary heart price, edema, lowered train capability, usually battle with easy each day actions and are often hospitalized. For a lot of, these signs considerably cut back their high quality of life. Identified causes of coronary heart failure embrace ischemic coronary heart illness and myocardial infarction (coronary heart assault), hypertension, valvular coronary heart illness, inflammatory ailments of the center corresponding to myocarditis and cardiomyopathies, anti-cancer therapies, and inherited metabolic ailments.
Coronary heart failure stays a number one reason for morbidity and mortality worldwide and persists as a rising well being and financial burden. In america alone, 6 million individuals over the age of 20 reside with coronary heart failure, and this quantity is projected to extend to greater than 8 million by 2030. The full annual price attributed to coronary heart failure is projected to extend to $69.8 billion by 2030.
Complete deaths attributed to coronary heart failure yearly in america have been reported within the vary of 86,000 to greater than 300,000, and hospitalizations vary from 800,000 to 1.3 million. The five-year mortality price for these with coronary heart failure has been reported at 52.6 % total.
Cardiol is growing CRD-38, a novel proprietary drug formulation designed to ship cannabidiol by subcutaneous administration. They’re endeavor IND-enabling actions to assist scientific analysis of CRD-38 as a therapeutic technique in coronary heart failure care – a number one reason for demise and hospitalization within the developed world, with related healthcare prices in america exceeding $30 billion yearly.
In 2023, Cardiol introduced optimistic examine outcomes from one in all its worldwide collaborating analysis facilities indicating how the CRD-38 subcutaneous formulation slowed will increase in physique weight and coronary heart weight and prevented will increase in key cardiac inflammatory and transforming markers in a mannequin of coronary heart failure with preserved ejection fraction.
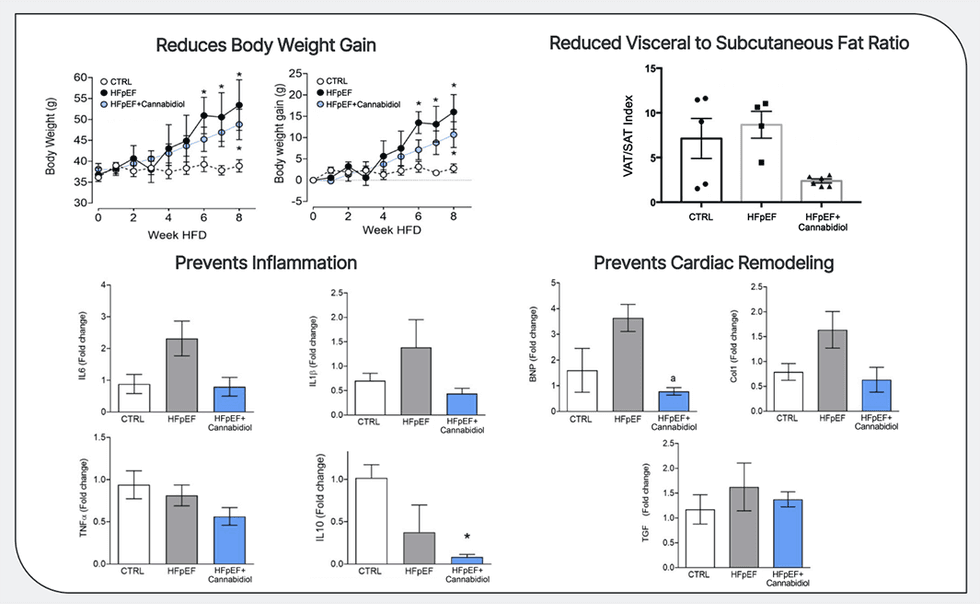
Printed third-party analysis has proven that cannabidiol reduces inflammatory activation of the endothelial lining of blood vessels and aids endothelial vasorelaxation, leading to improved blood circulate. Cannabidiol has additionally been proven to attenuate a number of measures of irritation in fashions of diabetes, a typical comorbidity in coronary heart failure sufferers, and to scale back myocardial fibrosis in a mannequin of inflammatory coronary heart illness.
Cannabidiol is lipid soluble, nearly insoluble in water, extremely delicate to deactivation within the liver by way of first-pass metabolism when taken orally and is quickly cleared from the physique. This ends in a low total bioavailability when taken orally. Cardiol’s subcutaneously administered drug formulation is designed to reduce first-pass metabolism, optimize and preserve blood ranges of the drug, and goal irritation and elevated fibrosis within the coronary heart. Cardiol believes that overcoming the low bioavailability points related to cannabidiol will considerably broaden the therapeutic potential of this molecule.
Cardiol Therapeutics’ Key International Analysis and Medical Collaborators
Cardiol is working along with world-class researchers and clinicians at worldwide facilities of excellence to leverage their experience in drug improvement, experimental execution, irritation and fibrosis, the therapy of cardiovascular ailments, and scientific trial protocol design. The collaborations present optimum recommendation and data platform in pursuit of Cardiol’s function: to heal the center with progressive science.

Administration Workforce
David Elsley – President, Chief Government Officer, and Director
David Elsley is the founder and CEO of Vasogen and has an MBA diploma. Elsley has over 25 years of expertise growing, financing, and managing all elements of company improvement in biotechnology and high-growth organizations. Elsley based Vasogen, a biotechnology firm targeted on the analysis and industrial improvement of novel therapeutics for the therapy of coronary heart failure and different inflammatory circumstances. Elsley assembled a group of administration, administrators and scientific advisors comprising business professionals and thought leaders from North America and Europe.
Dr. Andrew Hamer – Chief Medical Officer and Head of Analysis and Growth
Dr. Andrew Hamer has an MBChB diploma. He’s the previous govt director at Amgen, liable for main world improvement of Repatha®. Hamer is the previous chief heart specialist at Nelson Hospital, New Zealand. He has over 19 years of expertise working towards cardiology and inside drugs.
Chris Waddick – Chief Monetary Officer and Director
Chris Waddick has an MBA diploma, is a chartered skilled accountant, and is a licensed administration accountant. He has over 30 years of expertise in monetary and govt roles within the biotechnology and vitality industries. Waddick is the previous chief monetary officer and chief working officer of Vasogen Inc.
Bernard Lim – Chief Working Officer
Bernard Lim has over 30 years of expertise within the life sciences business, spanning biotechnology, diagnostics, medical units, and high-technology firms. He’s the founder and CEO of a extremely profitable drug supply firm that he led from analysis and improvement via to commercialization, and facilitated its eventual acquisition by Eli Lily. Lim is a chartered engineer per UK requirements and is a member of the Establishment of Engineering and Expertise.
Andrea B. Parker – Senior Director of Medical Operations
Dr. Andrea Parker is the previous chief scientific officer at Peter Munk Cardiac Heart, College Well being Community. Parker is a scientific epidemiologist with greater than 30 years’ expertise in scientific trials design, administration, and execution in business and tutorial settings.
John A. Geddes – Vice-president, Enterprise Growth
John Geddes has over 25 years of expertise within the healthcare business, comprising roles inside pharmaceutical, biotechnology, scientific diagnostics, and life science analysis know-how firms. Geddes has an MBA diploma and is the previous company senior director of enterprise improvement at Luminex Company, a DiaSorin Firm.
Anne Tomalin – Director of Regulatory and High quality
Anne Tomalin is the founding father of CanReg and TPIreg, regulatory corporations beforehand bought to Optum Perception and Innomar Methods, respectively. Tomalin is an knowledgeable in regulatory affairs in Canada, america, and Europe.
Blagoya Ristevski – Director of Chemical Engineering and Manufacturing
Blagoja Ristevski graduated with a BSc in chemical engineering and inorganic chemistry from the College of Ss ‘Cyril and Methodius’, Skopje, Macedonia, and pursued postgraduate analysis on pure biopolymers as drug carriers at King’s Faculty London, UK. For 20 years, he was concerned within the analysis and discovery of lively drug molecules, drug supply applied sciences, and manufacturing methodologies for completed drug merchandise at a number of biopharmaceutical firms.
Board of Administrators
Guillermo Torre-Amione – Chairman
Guillermo Torre is the president of TecSalud tutorial medical heart and faculty of the Instituto Tecnológico y de Estudios Superiores de Monterrey (ITESM), Mexico. He’s the previous director of Cardiac Transplantation on the Houston Methodist DeBakey Coronary heart & Vascular Heart.
Jennifer M. Chao – Director
Jennifer M. Chao has over 25 years of expertise within the biotech and life sciences industries targeted totally on finance and company technique. Chao is managing accomplice of CoreStrategies Administration, an organization she based in 2008 to offer transformational company and monetary methods to biotech/life science firms for maximizing core valuation.
Peter Pekos – Director
President and CEO at Dalton Pharma, Peter Pekos has broad expertise in analysis, improvement, and commercialization of prescribed drugs, merchandise, and companies.
Colin Stott – Director
Colin Stott has over 30 years of expertise in pre-clinical and scientific improvement, with particular experience within the improvement of cannabinoid-based medicines. Stott is the chief working officer of Alterola Biotech Inc. and the previous scientific affairs director, worldwide, and analysis and improvement operations director for GW Prescribed drugs, a world chief within the improvement of cannabinoid therapeutics.
Teri Loxam – Director
Teri Loxam has over 25 years of expertise within the pharmaceutical, life sciences and TMT industries with numerous roles spanning technique, investor relations, finance and communications.. Loxam would be the chief monetary officer of Compass Pathways plc (Nasdaq: CMPS), a biotechnology firm devoted to accelerating affected person entry to evidence-based innovation in psychological well being.
Michael Willner – Director
Michael Willner has practiced as an lawyer and a licensed public accountant. He graduated from Emory College Regulation Faculty as a member of the Emory Regulation Assessment. Subsequently, he practiced actual property and company legislation with New York Metropolis-based Milbank, Tweed, Hadley & McCloy, one of many nation’s most distinguished worldwide legislation corporations. Earlier than his authorized profession, Willner was employed by the previous Arthur Andersen & Firm, a nationwide accounting agency, the place he practiced within the tax division.
Scientific Advisory Board
Dr. Paul Ridker
Dr. Paul Ridker is director of the Heart for Cardiovascular Illness Prevention, a translational analysis unit at Brigham and Ladies’s Hospital in Boston (BWH). A cardiovascular drugs specialist, he’s additionally the Eugene Braunwald Professor of Medication at Harvard Faculty of Medication (HSM). Ridker obtained his medical diploma from HSM after which accomplished an inside drugs residency and a cardiology fellowship at BWH. He’s board licensed in inside drugs. Ridker’s scientific pursuits embrace coronary artery illness and the underlying causes and prevention of atherosclerotic illness. He’s the creator of over 900 peer-reviewed publications and critiques, 64 guide chapters, and 6 textbooks associated to cardiovascular drugs.
Dr. Bruce McManus
Dr. Bruce McManus is a professor emeritus of the Division of Pathology and Laboratory Medication on the College of British Columbia. He has served as CEO of the Heart of Excellence for Prevention of Organ Failure (PROOF Heart), director of the UBC Heart for Coronary heart and Lung Innovation, and scientific director of the Institute of Circulatory and Respiratory Well being, CIHR. McManus obtained BA and MD levels from the College of Saskatchewan, an MSc from Pennsylvania State College, and a PhD from the College of Toledo. McManus pursued post-doctoral fellowships on the College of California, Santa Barbara in environmental physiology and on the Nationwide Coronary heart, Lung, and Blood Institute in Bethesda. McManus served as MD in cardiovascular and pulmonary pathology, and accomplished residency coaching on the Peter Bent Brigham Hospital, Harvard College, in Inside Medication and Pathology.
Dr. Joseph Hill
Dr. Joseph Hill is a professor of inside drugs and molecular biology, chief of cardiology at UT Southwestern Medical Heart, in Dallas, and is the director of the Harry S. Moss Coronary heart Heart. Hill holds each the James T. Willerson, MD, distinguished chair in cardiovascular ailments, and the Frank M. Ryburn Jr. Chair in Coronary heart Analysis. He graduated from Duke College with an MD and a PhD in 1987. Hill’s PhD dissertation analysis was within the area of cardiac ion channel biophysics. He then labored for 5 years as a postdoctoral fellow on the Institut Pasteur in Paris, learning central and peripheral nicotinic receptors. He subsequent accomplished an inside drugs internship and residency, in addition to a scientific cardiology fellowship, on the Brigham and Ladies’s Hospital, Harvard Medical Faculty.

